construct the orbital diagram for arsenic|lewis dot structure for arsenic : Tagatay Hund’s principle is a rule that helps to determine how electrons are distributed in orbitals when multiple orbitals of the same energy . Tingnan ang higit pa Welcome to FB777, the premier online casino destination in the Philippines, where excitement and rewards await you at every turn. With our extensive selection of games, generous bonuses, and top-notch service, FB777 promises an unparalleled gaming experience like no other.
PH0 · orbital diagram for k
PH1 · lewis dot structure for arsenic
PH2 · lewis dot for as
PH3 · how to do orbital diagrams
PH4 · carbon orbital diagram
PH5 · arsenic quantum numbers
PH6 · arsenic lewis structure
PH7 · arsenic electron dot structure
PH8 · Iba pa
gov.je. Information and public services for the Island of Jersey. L'înformâtion et les sèrvices publyis pouor I'Île dé Jèrri
construct the orbital diagram for arsenic*******Orbital diagrams are usually represented by boxes. Each box represents an orbital and the arrows within the box represent the position of the electron. The boxes are arranged in order of energy of the orbitals. The lowest energy orbitals are closest to the nucleus and the higher energy orbitals . Tingnan ang higit pa
The electrons of the atomrevolve around the nucleus in a certain circular path. These circular paths are called orbit (shell). Again, atomic energy shells are subdivided . Tingnan ang higit pa
The arsenic orbital notation is a shorthand system designed to represent the exact positions of the electrons in the arsenic atom. This is similar to electron configuration, but numbers are used instead of boxes to represent the positions of the . Tingnan ang higit pa
Hund’s principle is a rule that helps to determine how electrons are distributed in orbitals when multiple orbitals of the same energy . Tingnan ang higit paConstruct the orbital diagram for arsenic. Answer Bank Energy. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer. .
To write the orbital diagram for the Arsenic atom (As) first we need to write the electron configuration for just As. To do that we need to find the number o. Steps. Find electrons. Periodic table | Image: Learnool. The atomic number of arsenic represents the total number of electrons of arsenic. Since the atomic number of .
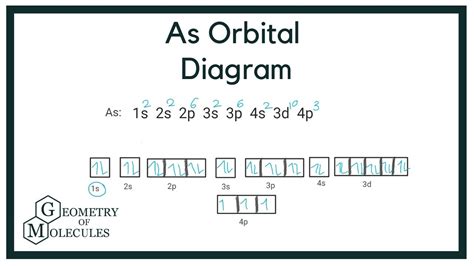
On the basis of pairs and unpaired electrons, an orbital diagram is formed. In case of Arsenic there are 3 unpaired boxes of electrons of 4p 3 and rest 30 electrons in the pairs. Period Table. Check . 3.73K subscribers. Subscribed. 3. 291 views 1 year ago Electron Configuration. In this video, we will see what orbital diagrams are, the rules that we .construct the orbital diagram for arsenic lewis dot structure for arsenic Aufbau principle. First, find electrons of arsenic atom. Periodic table | Image: Learnool. The atomic number of arsenic represents the total number of electrons of .We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams .The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Arsenic atomic orbital and chemical bonding .
Write the electron configuration of arsenic (As). Draw the orbital diagram for an atom with an electron configuration of 1s22s22p63s23p3. Draw the orbital diagram for an atom with an electron configuration of 1s22s22p63s23p64s23d7. Draw the orbital diagram for an atom with an electron configuration of 1s22s22p5.
Step 1: Understand the Basics of Orbital Diagrams. Before constructing the orbital diagram for arsenic, it is important to have a solid understanding of the basics of orbital diagrams. Orbital diagrams are visual representations of the electrons in an atom’s orbitals. They show the specific arrangement of electrons in each energy level and . The arsenic electron configuration, represented as [] 4s 2 3d 10 4p 3 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 3, showcases the precise arrangement of electrons within the atom.This configuration can be determined through various methods, including the aufbau principle, periodic table organization, Bohr model representation, or orbital .Draw the atomic orbital diagram for nitrogen. Draw and explain the orbital diagram for Au+. Draw the orbital diagram for the valence shell of each of the following atoms: (a) C (b) P (c) V (d) Sb (e) Ru; Draw and explain the orbital diagram for phosphorus. Write orbital diagram for cd^{2+}. Draw and explain the molecular orbital diagram for NO2.This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Construct the orbital diagram for arsenic to show the full ground-state electron configuration. IO Answer Bank Energy. Here’s the best way to solve it.
Orbital Diagrams. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above.Orbital diagram. Arsenic electron configuration. ← Electronic configurations of elements. As (Arsenic) is an element with position number 33 in the periodic table. Located in the IV period. Melting point: 613 ℃. Density: 5.72 g/cm 3 . Electronic configuration of the Arsenic atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p .Construct the orbital diagram for arsenic. 4p LID 3d 4s CLL Answer Bank 3p Energy 11 3s CDI 2p 2s 1s This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Due to this, only two electrons with opposite spins can be paired in an orbital, Part The electron configuration of arsenic, As, is 1s 2s 2p 3s 3p"4393404p" The orbitals are represented schematically as a box, and each electron is represented by an arrow. Complete the orbital diagram for As. Drag the appropriate labels to their respective .
construct the orbital diagram for arsenic About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright .Question: Construct the orbital diagram for arsenic. Energy 4p 3d 4s 3p 3s 2p 2s 1s ODO DOOOO O ODO O Ooo Answer Bank 14. Construct the orbital diagram for arsenic. Energy 4p 3d 4s 3p 3s 2p 2s 1s ODO DOOOO O ODO O Ooo Answer Bank 14. Show transcribed image text. There are 2 steps to solve this one.Construct an energy level diagram showing all orbitals for the hydrogen atom up to n=5, labeling each orbital with its appropriate quantum numbers. How many different orbitals are in each shell? State which of the following orbitals cannot exist according to the quantum theory: 2s, 2d, 3p, 3f, 4f, and 5s. Construct the orbital diagram for arsenic to show the full ground-state electron configuration. 01:07. Draw a partial (valence-level) orbital diagram, and write the condensed ground-state electron configuration for each: (a) Ti (b) Cl (c) V. 00:21.
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Construct the orbital diagram for arsenic. Answer Bank Energy Biotoboo. Here’s the best way to solve it. The construction of the orbital diagram of the Arsenic is given b ..Electronic Structure of Atoms. Orbital Diagrams. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. Each box represents one orbital, and each arrow indicates one electron. This is a way of showing the electron configuration of the atom. For example, the orbital diagram of Li can be .Write the orbital diagram for the ground state of the arsenic atom. Give all orbitals. Write the charge and full ground-state electron configuration of the monatomic ion most likely to be formed by P. Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element.

An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below.Construct the orbital diagram for arsenic. Energy 4p 3d 4s 3p 3s 2p 2s 1s 00000 OOO OOO. Construct the orbital diagram for arsenic. Energy 4p 3d 4s 3p 3s 2p 2s 1s 00000 OOO OOO. BUY. Chemistry & Chemical Reactivity. 10th Edition. ISBN: 9781337399074. Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel.
Complete career NBA stats for the Dallas Mavericks Point Guard Luka Doncic on ESPN (PH). Includes points, rebounds, and assists.
construct the orbital diagram for arsenic|lewis dot structure for arsenic